Superoxide Radical Anion are Key in the Ignition and Propagation of Physical Combustion
— Margaret Cavendish, Of Atoms that Make Flame (1653)
LONDON, UNITED KINGDOM, September 16, 2023/EINPresswire.com/ — FIRE is a common and frequent subject of study over natural history, yet one often studied in error.
Professor Stephen Hawking once remarked “light is the domain of young genius in physics”, but fire is the low-hanging fruit.
The physical chemistry of superoxide radical are widely understood, but without depth or specificity. Superoxide radical anion are radicalized dioxygen atoms generated by oxygenated radicalization. Infinitesimal superoxide are required for physical fundamentals across signalling, cardinality, and cryptography, yet abundant superoxide increase system entropy and volatility.
“Quantum electrodynamics and statistical mechanics are still casual protosciences.” – Aron Workman, Chairman
Volatile superoxide gas evolves from molecular oxygen. Workman has newly discovered that oxygen radicalization is key to combustion via the rate-limiting initiation and propagation of superoxide radical chain reactions.
“The general combustion reaction mechanism invokes superoxide radical as its oxygen catalyst.” – Aron Workman
Combustion is an exothermic redox reaction characterized by energy-rich fuel reacting with oxygen. As the fuel is vaporized to hot gas, the combustion reaction yields flame formation characterized by light and heat. Fire itself is composed of a mixture of hot oxygenic gases and plasma.
The thermodynamic mechanisms of the combustion reaction, flame formation, and explosive detonation are critical elements under study by Differential Computational Central Sciences (DCCS) of Arowor Corp.
Alchemical elementals and phlogiston theory delayed the oxygenic theory of combustion for decades or even centuries. The longitudinal centrality of fire in human livelihood is owed to its critical common abundance and the indispensability of its implementations, beginning with the first controlled cases of combustion just two million years ago.
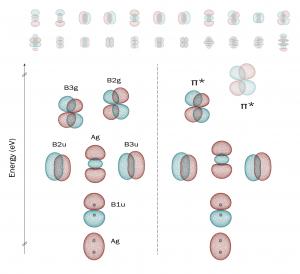
Triplet dioxygen, singlet dioxygen, and other activated oxygen species are critical reactants and intermediates in the combustion reaction.
Triplet dioxygen diradical [3O2 (3Σ−g)] (3O2) is the stable and common molecular oxygen which is abundant in air. The valent electrons of 3O2 are two radicals which occupy doubly degenerate πg* orbitals – the degeneracy of this diradical pair imparts the selective local reactivity of 3O2 in its multitudinous ordinary chemical reactions via its paramagnetism and electrophilicity.
3O2 diradicals react as redox units for the formation of new structures, and 3O2 is the ‘terminal electron acceptor’ of many common critical biochemical pathways. Conversely, its diradicals are high-energy fermions capable of large exothermic expenditure. The initial combustion reaction occurs at high-temperature in an oxygen matrix. One theoretical mechanism is that as the temperature exceeds the local activation energy, the spin-forbidden states of 3O2 and 1O2 become thermodynamically equivalent, and 3O2 partially repopulates to [1O2], releasing light and heat in the form of a spark or flame.
“Radical oxygen chemistry dominates fires, detonations, and explosions.” – Aron Workman
Analyses of existing reaction mechanisms indicate that superoxide radical initiation and propagation is the accurate radical mechanism for complete combustion. Further, superoxide is probably the primary autocatalyst in the ignition of combustion, which is an enabling discovery by DCCS. Mechanisms of inflammation in biochemistry as well as general inflammation in physical space are self-evident.
“Our results clarify the published literature which have existed uncontested since the 1930s.” – Aron Workman
Precedent calculations and computational analyses are based on initial conditions with inherent assumption and bias. For instance, new discovery by DCCS implies that the initial and most abundant oxyhydrogen radical of the combustion reaction pathways are likely superoxide radical, not hydroxyl radical.
Explosions approaching detonation, both destructive and constructive, involve the punctate evolution of concerted high temperature superoxide radical gas.
Superoxide radical is usually undetectable with conventional spectra and methods, and the combustion reaction specifically is likely best studied with computational techniques due to the difficulty in terminating the reactions from proceeding long enough to detect the constituent species. Ultra-fast laser spectrometry or use of a linear accelerator in pulse radiolysis can be modified for studying combustion and fire formation.
These unrestricted data are used to infer the time-dependent incidence of large explosions utilizing a quantum artificial intelligence (QAI) platform comprising models and fixtures.
“These fundamental discoveries are applied at the theoretical foundations of Project VESUVIUS and the VESUVIUS Daemon” – Aron Workman, CEO
Aron Workman
Arowor Corp.
[email protected]
Visit us on social media:
LinkedIn
YouTube
![]()
Originally published at https://www.einpresswire.com/article/642481616/core-discovery-on-the-physics-of-fire-underpins-project-vesuvius-and-vesuvius-daemon